Explore Evolution ignores mutations in non-protein-coding cis-regulatory sequences (CREs). Furthermore, research has shown that mutations in the coding regions and in CREs of the genetic toolkit are both responsible for evolutionary change.
Evolutionary developmental biologists argue that mutations in members of the genetic toolkit will be most important for driving morphological change during the evolution of animals. Many genetic toolkit genes, such as Ultrabithorax, a member of Hox gene family, regulate developmental pathways by turning other genes on or off. Hox proteins bind to specific DNA sequences in CREs and control whether or not RNA is synthesized from the adjacent gene.
Explore Evolution claims:
Because hox genes are so important for coordinating the activities of the cell, some researchers think that mutations in these genes can cause large-scale changes in the structure of an organism. Many neo-Darwinists think that influencing the "coach" (hox genes) through mutation can provide a new source of beneficial variation. Critics aren't so sure. They note that, precisely because hox genes are so influential, no experimental mutations in hox genes (so far) have proven helpful to the organism.Explore Evolution, p. 109
Explore Evolution latches onto the current circumstance that experimental mutations in the Hox genome have yet to result in an advantage to an organism. Then they invoke anonymous "critics" and suggest that evolution does not involve Hox gene mutations. This is the typical creationist ploy of identifying an experimental shortcoming to generate "doubts" about evolution. This time, they raise the bar rather high for evolutionary developmental biologists. Given the hundreds of millions of years in which evolution has been optimizing morphology, Explore Evolution injects "doubt" because researchers have yet to make a further structural improvement in a decade or so of research.
However, we can investigate whether natural mutations in the protein-coding regions of Hox genes have been responsible for changes in body plans. To understand how the changes a Hox gene could result in evolutionary change, evolutionary developmental biologists compare the functions of Hox genes in different organisms. For example, Ultrabithorax is known to repress limb formation in insects, such as fruit flies. However, the abdominal segments of crustaceans, such as brine shrimp, express Ultrabithorax and also have limbs. So, Ultrabithorax is able to repress limb formation in insect embryos but not in crustacean embryos. Notably, a major feature of the insect body plan is their lack of abdominal limbs, unlike crustaceans. Thus the difference between crustacean and insect Ultrabithorax function in repressing limbs could play an important role in arthropod evolution.
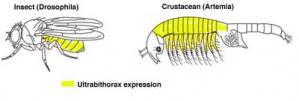 Ultrabithorax in Insect and Crustacean Limbs
Ultrabithorax in Insect and Crustacean Limbs
The comparison of a genetic toolkit gene, Ultrabithorax in insects, crustaceans and velvet worms has established why insects lack abdominal limbs (Galant et al., 2002; Ronshaugen et al., 2002). Changes in sequence at the tail end of Ultrabithorax protein have enabled Ultrabithorax to repress abdominal limbs in insects.
 Ultrabithorax
Ultrabithorax Genetic experiments in fruit flies has shown that crustacean Ultrabithorax does not repress embryonic limbs, unlike fruit fly Ultrabithorax. An important reason for the difference in Ultrabithorax function is that all modern insects have a "QA motif" - a short 15 amino acid sequence (QAQAKAAAAAAAAAA)at the tail of Ultrabithorax protein. Arthropods, such as millipedes and crustaceans, have a different type of sequence at the tail of Ultrabithorax which has a number of serines (S) and threonines (T). In a functional analysis in fruit flies, Ronshaugen et al. show that the replacement of a few of the serines (indicated by * in diagram below) in the tail of a crustacean Ultrabithorax renders it capable of repressing limbs.
As McGinnis and colleagues explain:
Previous studies led us to propose that gain and loss of transcriptional activation and repression functions in Hox proteins was a plausible mechanism to diversify morphology during animal evolution(12). Here we show that naturally selected alteration of the Ubx protein is linked to the evolutionary transition to hexapod limb pattern.Ronshaugen et al., "Hox protein mutation and macroevolution of the insect body plan." (2002) Nature 415, p. 914
The evidence for the importance of Hox gene mutations in morphological evolution certainly extends beyond creating a four-winged fruit fly.
